
Advancing Clinical Data Transformation: Industry Outlook 2024
Overview
Clinical data transformation is one of healthcare organizations’ top strategic priorities. Its success is largely influenced by the ways in which organizations address artificial intelligence (AI) adoption, external data use, and cycle time pressures related to electronic data capture (EDC) implementation and insight generation.
The push for clinical data transformation is happening in a broader industry context defined by an emphasis on patient centricity, decentralization of data collection, increasing clinical trial complexity, technology advances, and an ongoing drive for efficiency.
eClinical’s Industry Outlook 2024 report—which incorporates responses from professionals working across clinical operations, data management, statistical programming, and biometric functions at over 50 biopharmaceutical sponsor organizations—sheds light on the trends, practical experiences, and important implications for clinical data transformation in the year ahead.
Context
eClinical Solutions’ Katrina Rice, chief delivery officer, biometrics services, Diane Lacroix, vice president of clinical data management, and Sandy Ng, vice president of data science, discussed findings and insights from eClinical’s Industry Outlook 2024 report, their implications for trial sponsor organizations and CROs, and predictions for the adoption of AI/machine learning (ML) models in the industry in 2024 and beyond.
Key Takeaways
Organizations are using different strategies to handle increasing trial and data complexity.
Asked to identify the top three most effective strategies their organizations are using to handle greater clinical trial and complexity, survey respondents pointed to adapting processes and workflows (65%), incorporating advanced analytics tools (50%), and implementing skills development and training programs for staff (45%).
Figure 1: Most effective strategies being used to handle greater trial and data complexity
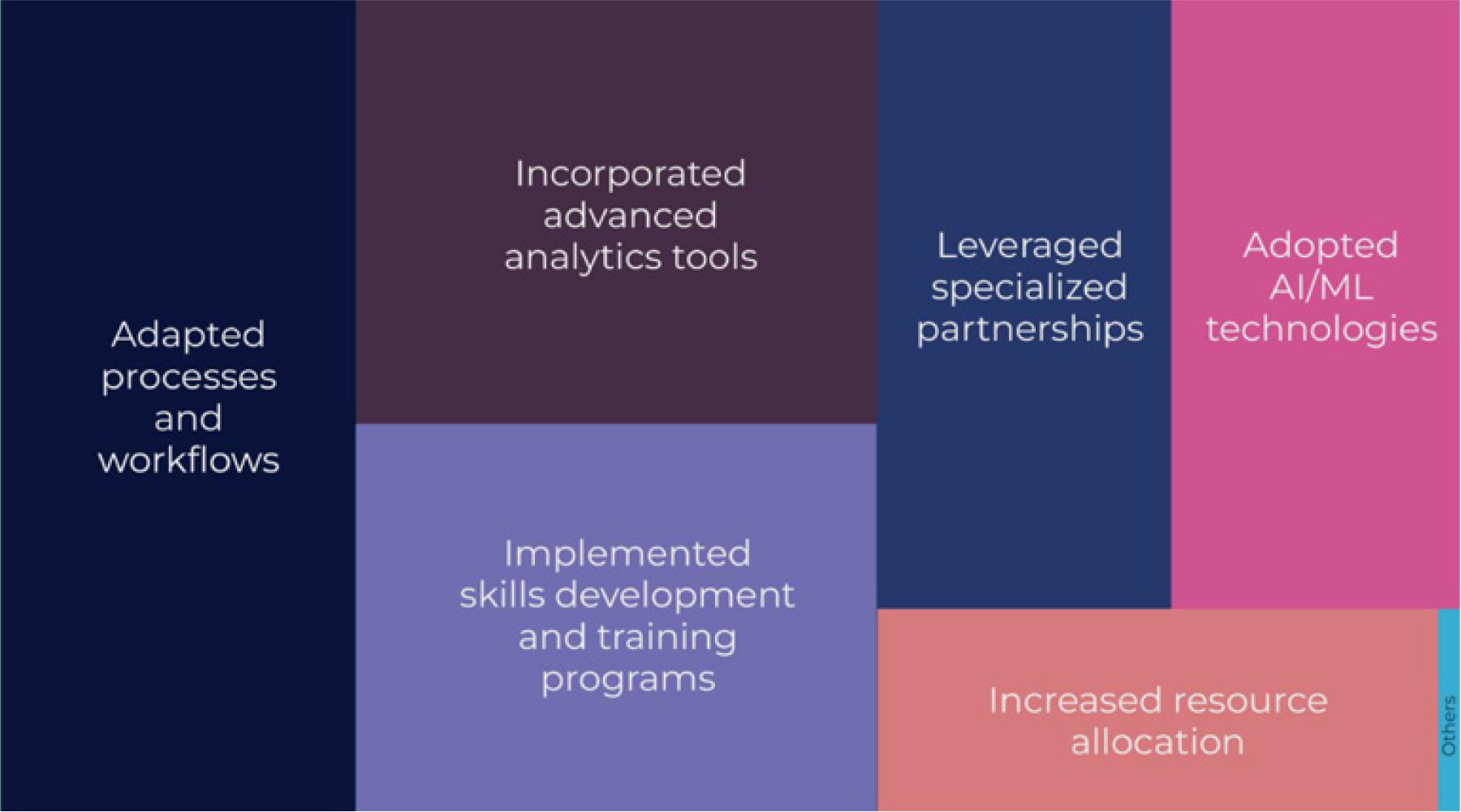
Strategies that ranked lower on respondents’ lists of current strategies included adopting AI/ML technologies, leveraging specialized partnerships, and increasing resource allocation. This makes sense because despite the generalized excitement around the future application of AI/ML technologies, organizations naturally focus on areas which are within their immediate control and can deliver predictable ROI, such as adapting internal processes, Lacroix said.
From that perspective, and in the context of continued R&D productivity pressures, it also makes sense that pharmaceutical sponsors are prioritizing upskilling the existing workforce rather than simply allocating more resources to recruitment.
If you continue to do resource hiring and adding more people, you aren’t going to easily be able to get cost savings. But if you focus on training and upskilling the resources you have, while looking at how you adapt your processes and workflows, that may lead to cost reductions.
Katrina Rice, eClinical Solutions
“Focusing on more efficient processes and upskilling of staff tampers down the need to bring on more people to run clinical trials,” Lacroix added. Lacroix highlighted that the new skills clinical data professionals need today include having a true understanding of the data being collected rather than simple data cleaning, employing risk-based approaches, learning how to operationalize complex data collection protocols, and moving away from an EDC-centric approach to a patient-centric approach.
Still, the adoption of AI/ML technologies is anything but a secondary priority.
AI and ML technologies are expected to have an outsized impact on efficiency and outcomes.
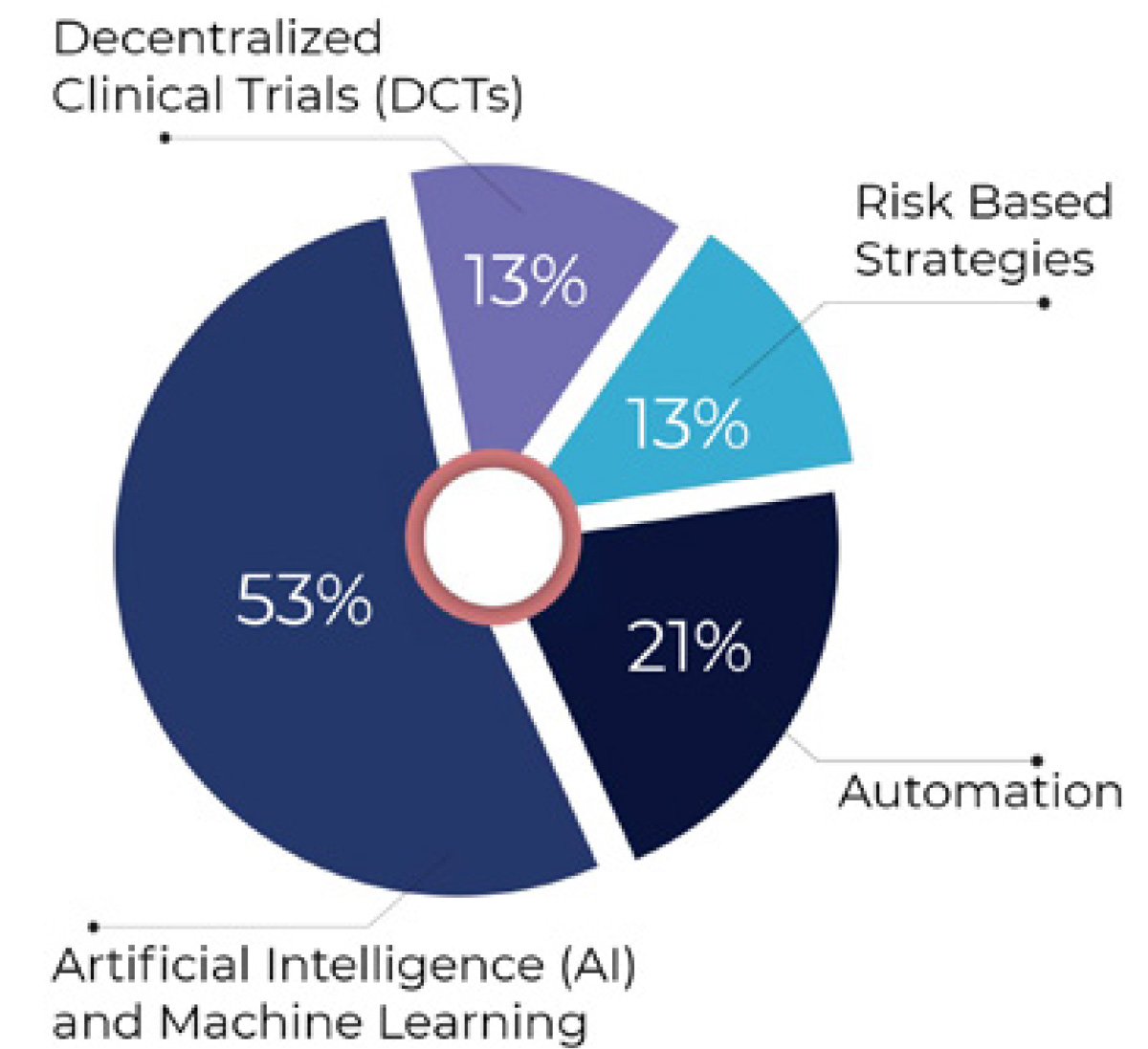
Although adopting AI/ML technologies was not among survey respondents’ top three currently deployed strategies for addressing the growing complexity of clinical trials and data management, it was identified by 53% of respondents as the leading trend expected to have the greatest impact on efficiency and outcomes over the next 12 months.
Other industry trends perceived as having the greatest impact on efficiency and outcomes in the next 12 months were adoption of automation (21%), risk-based strategies (13%), and decentralized clinical trials (13%).
“AI can be applied to automation, to decentralized clinical trials (DCTs), and to risk-based strategies, so there is not a clear delineation between these four priorities that we are seeing here,” Ng said. She added that to date her team at eClinical has mostly focused on using AI for data monitoring and data review projects but is soon planning to also begin leveraging the capabilities of AI/ML for data transformation and fraud detection.
Figure 2: Top industry trends seen as having the greatest impact on efficiency and outcomes in next 12 months
A lot of people are dipping their toes into AI/ML and trying to figure out how impactful this will be in our industry.
Sandy Ng, eClinical Solutions
Lacroix noted that one of the biggest benefits of adopting AI/ML technologies for data review is that it allows clinical data management teams to focus on cases that cannot use rule-based automation because they require higher levels of critical thinking, decision making, and human supervision.
The incorporation of such technologies is likely to have a huge upside for the clinical data ecosystem by also improving anomaly detection in lab results, consistency in reporting adverse events, real-time data monitoring, informed consent, trial design, predictive modeling, and patient recruitment.
The industry believes that AI/ML is going to pack a huge punch in the next 12 months.
Diane Lacroix, eClinical Solutions
However, as important as AI and ML are, at this point, few organizations (just 7%) have integrated AI/ML across one or multiple applications, and most organizations are still in the exploratory stages of integrating AI and ML into clinical data workflows.
Figure 3: The degree of integration of AI and ML approaches into clinical data workflows

Reducing cycle times across clinical data management remains a challenge.
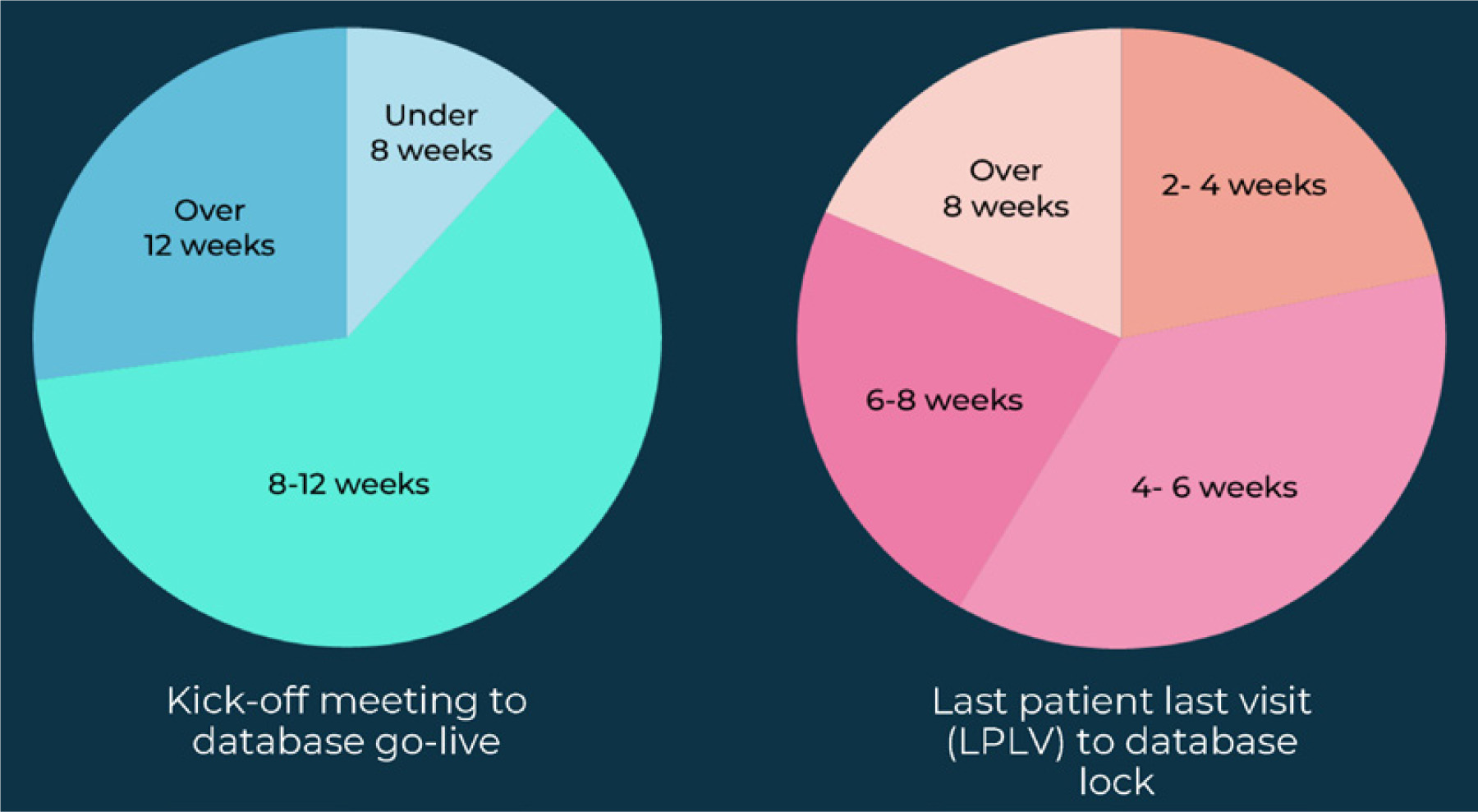
Industry-average cycle times for data management solutions vary widely across organizations. Respondents to the Industry Outlook survey found that at most organizations, the time between a typical kick-off meeting to “database go-live” averages 8-12 weeks.
For a typical last patient last visit (LPLV)-to-database lock cycle, organizations often averaged 6-8 weeks or over 8 weeks. Few respondents (less than 25%) take 2-4 weeks.
Figure 4: Industry cycle times across the data life cycle
These variances are often a function of the prolonged back-and-forth in the form of meetings, conversations, and decision making that takes place between sponsors and service providers, whose input data management teams need before rolling out data solutions.
It’s a lot around the back and forth, the questions, the understanding that feeds into those cycle times. It takes a village to make sure that we can [shorten] those collectively.
Katrina Rice, eClinical Solutions
“Reducing cycle times is not a data acquisition team or data management team-only responsibility; our sponsors play a huge role in these activities. We know an 8-to-12-week timeline is not build time, it’s back-and-forth time,” Lacroix said.
Aligning stakeholders early on, getting buy-in as to the outcomes we’re trying to achieve, and setting expectations and goals is really important in shortening cycle timelines.
Diane Lacroix, eClinical Solutions
“It is a shared relationship,” Rice said, commenting that clients often pose questions about how fast eClinical can deliver a given outcome. “I always like to say, ‘We can be as fast as you are.’”
To help companies reduce cycle times and enhance quality, eClinical has developed purpose-built AI/ML models for data review applications that can trim the total volume of records that need to be reviewed by 70-90%. Ng noted that other broader AI/ML applications can also help reduce cycle times and improve efficiency by informing trial design, identifying target endpoints, and predicting dropout rates.
Conclusion
With clinical trial and data complexity continuing to challenge trial sponsors and CROs to update internal data management processes, being able to see around the corner and know what to expect can help them prepare for these shifts.
The integration of AI/ML models and advanced analytics capabilities within those processes and workflows is inevitable and likely to accelerate—and one way that companies can position themselves for success is by developing benchmarks and metrics that capture the ROI of such integrations. The probable increase in mergers and acquisitions in the life sciences industry is also expected to propel some changes. “We’re going to see way more mergers and acquisitions, which is going to force the need to bring a lot more data and data sharing knowledge together across organizations,” Rice said.
Last but not least, the interest and energy surrounding AI/ML models may well turn out to be a self-fulfilling prophecy, driving their faster integration into data management workflows and the clinical trial ecosystem as a whole.
My prediction is that by the end of 2024, there will be even more excitement about AI than there is at the beginning of the year.
Sandy Ng, eClinical Solutions
Authors

Diane Lacroix is a data management professional and leader with over 20 years in the pharmaceutical/CRO industry. As VP, Clinical Data Management at eClinical Solutions, Diane is responsible for leading the data management function including building effective process and implementation strategies to ensure that clients receive maximum value and quality from the elluminate driven data services that Diane’s team delivers. Diane’s career in data management has included numerous leadership roles both at service providers and on the sponsor side. She has worked on all aspects of global trials from study start-up through to database closure and submission leading and managing successful teams and projects to ensure high quality and on-time delivery of clinical data assets. Diane has deep expertise in the oncology and rare disease therapeutic areas and in data management technologies including EDC (Electronic Data Capture) systems and integration and analytics technology platform like elluminate.

Sandy Ng is the VP of Data Science at eClinical Solutions. Her team collaborates closely with internal and external stakeholders to bring humans-in-the-loop AI/ML solutions to life science problems. She holds a doctorate in engineering and is experienced with building data science products for cardiovascular and neurological applications at startup and midsized medical device companies and at academic medical centers. For the past five years, she has been focused on AI/ML applications to healthcare payer and provider data and clinical trials data.

Katrina Rice is an accomplished Chief Delivery Officer with an impressive career that spans over 25 years and includes advancement into increasingly demanding leadership roles. With a solid history of leading business transformations and managing global portfolios, she is as much at home scaling operations as she is in developing strategies that drive revenue growth. At eClinical Solutions, Katrina was recently promoted from Executive Vice President of Professional Services to Chief Delivery Officer. She has previously held various technical roles at Lockheed Martin Energy Group and Bayer. Katrina holds a Bachelor of Science degree in Computer Science from Alabama Agricultural and Mechanical University and a Master of Science degree in Computer Science with Advanced Applications from the University of New Haven. In her free time, Katrina is an active participant of Chief, a private network for women in senior leadership roles, Treasurer in her church where she oversees all financial aspects and a Member of the Delta Sigma Theta Sorority Incorporated, a not-for-profit organization dedicated to public service with an emphasis on programs that assist the African American community.
By submitting, you agree to the processing of your personal data by eClinical Solutions as described in our Privacy Policy.







