
Drivers for An Advanced Statistical Computing Environment (SCE)
As innovation in clinical development continues to accelerate and decentralized trial (DCT) models become more widely adopted, the volume of clinical trial data from a variety of sources continues to proliferate, creating both opportunities and challenges for life sciences organizations. While more advanced trial modalities such as DCT make trials more accessible and relieves patient burden, the volume and variety of study data collected adds to the complexity of managing clinical data workflows throughout the trial lifecycle. More complex multi-arm trials used for oncology products can also require enormous data and content stores, challenging the capabilities of legacy clinical data stores. Leveraging a modern technology solution, such as a statistical computing environment, is one way sponsors can get in front of these new clinical development challenges.
What is a Statistical Computing Environment (SCE)?
A Statistical Computing Environment (SCE) is a set of tools for computational processing of clinical data that provides a foundation for demonstrating rigor — which requires transparency, reproducibility, and adequate documentation — in the analysis and reporting of clinical trial results.
Computational Processing of Clinical Data
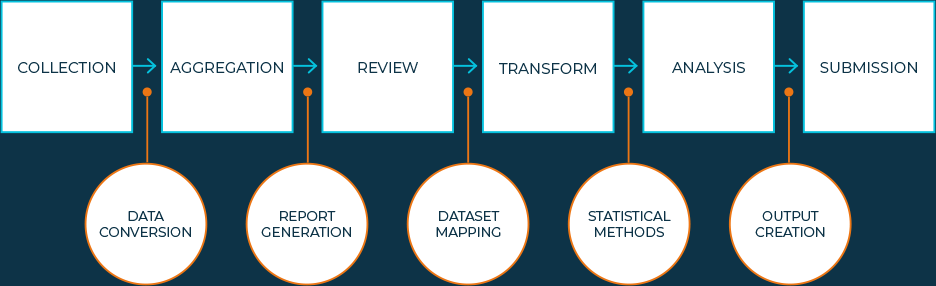
There are a variety of factors driving the need for an advanced computing environment within life sciences organizations. As computational complexity continues to increase — driven by factors including larger data sets, more sophisticated methods and the emergence of data science techniques — a high-performance computing environment is critical to support output generation. Moreover, a modern statistical computing environment provides the flexibility required to support highly specialized analytic needs and language-agnostic approaches. Additionally, because many organizations support global cross-functional teams, cloud-based solutions are becoming table stakes to ensure the availability of, and access to, real-time clinical data.
Historical Approaches to Statistical Computing
There are a variety of factors driving the need for an advanced computing environment within life sciences organizations. As computational complexity continues to increase — driven by factors including larger data sets, more sophisticated methods, and the emergence of data science techniques — a high-performance computing environment is critical to support output generation. Moreover, a modern statistical computing environment provides the flexibility required to support highly specialized analytic needs and language-agnostic approaches. Additionally, because many organizations support global cross-functional teams, cloud-based solutions are becoming table stakes to ensure the availability of, and access to, real-time clinical data.
SCE Adoption in Today’s Market
In a recent poll, 40% of respondents from mid-market and large sponsors have either purchased an SCE, are in the process of purchasing an SCE, or are building their own validated system. These results indicate that the value of a statistical computing environment is resonating within the market. As DCT and digital trials continue to evolve with larger and more complex data sets, we anticipate these dynamics shifting in the near future. Given the rapidly changing landscape, it seems likely that the 52% of organizations using standalone SAS and/or R will start moving towards purchasing an SCE in order to reduce administrative burden and keep up with the digital evolution of modern clinical trials.
2022 eClinical Solutions Industry Poll:
How sponsors are approaching statistical
computing needs
What’s your organization’s current solution for
statistical computing?
Outsource tasks or have no internal statistical computing

Don’t have an SCE but have standalone SAS and/or R

Have either purchased or are purchasing a commercial SCE

Have built or are building their own validated SCE
Read The Digital Statistical Computing Environment (SCE) Playbook for Clinical Development to further explore how sponsors can leverage an integrated Statistical Computing Environment (SCE) to get in front of new clinical development challenges to streamline the production of submission deliverables while increasing programming and analysis efficiencies.








