
Powering Effective Oversight: Creating Actionable Clinical and Operational Data Insights
The explosion of data
Over the past few years we have seen an explosion in the velocity, variety and volume of data within clinical trials and across the broader healthcare arena. We now have devices recording 24/7 readings, potentially millions of data points and up to 10 unique data sources per clinical trial. Historically, most trial data was captured directly within the EDC systems, however, this has decreased to ~30 – 40%. The need for robust solutions for effective oversight has never been so important.
The evolution of oversight solutions
Despite significant advances in clinical trial management and oversight solutions, in up to 50% of cases, excel and other trackers are still being used by sponsors and CRO’s to manage clinical and operational data. Siloed approaches for data ingestion, and limited data visibility, are no longer acceptable. A change in mindset and approach is now required to create meaningful and actionable insights.
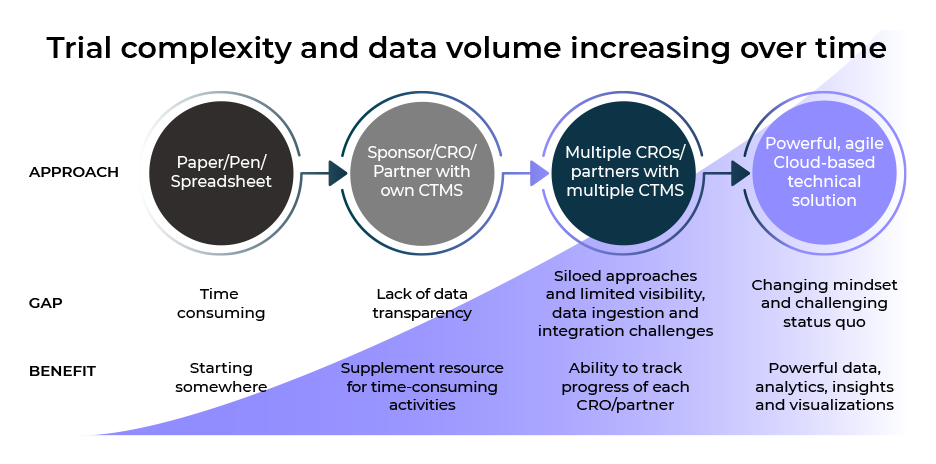
The challenge of complexity
The prevalence of more ‘agile’ or ‘hybrid’ clinical trials has been accelerated by the recent devastating pandemic. Whilst positive for patients, modern approaches for site identification/startup, consent, recruitment, Home Healthcare Nursing, remote monitoring, direct to patient IP shipping etc. in a virtual setting has huge benefits, however, it adds complexity for overseeing and managing clinical trials..
Additionally, our trial designs are becoming more complex. In Oncology, umbrella trials explore the same cancer type but with different gene mutations or biomarkers and basket trials look at new treatments or combinations that are tested across a range of cancer types that have similar mutations or biomarkers. The oversight of these, and other adaptive trials, requires timely access to the evolving data to make the right decisions and manage risk.
Modern Clinical Data Platforms must now be able to manage both ‘active’ and ‘passive’ clinical trial data. The increasing variety of unique data sources also creates challenges for sponsors and clinical operations professionals as they attempt to integrate, and make sense of all the information. They must be able to integrate data from multiple sources, and different clinical trial management systems (CTMS), with the flexibility and agility to provide near real-time access to data.
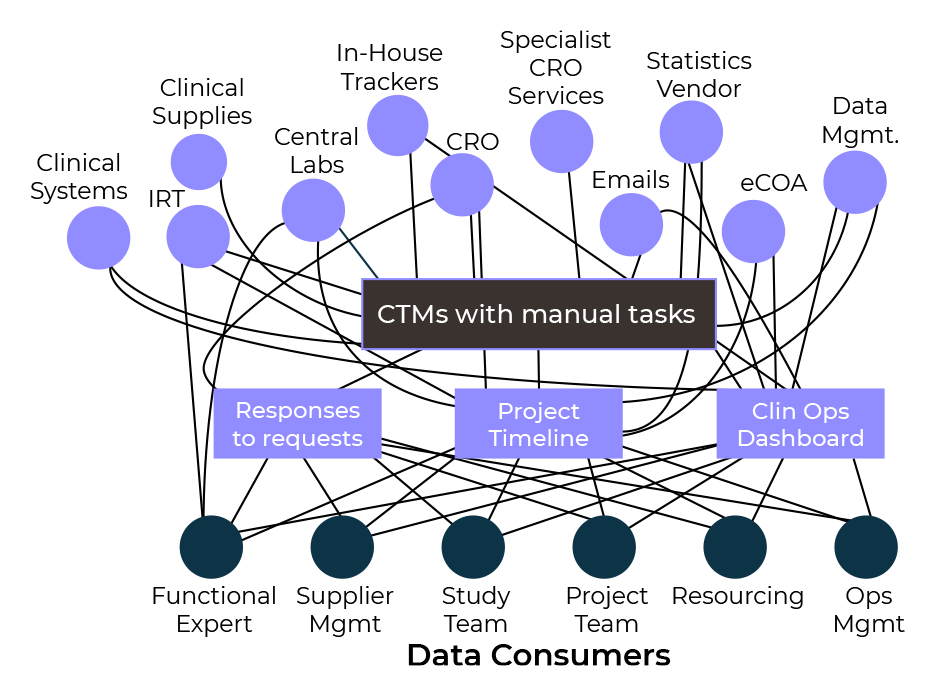

What role does a CTMS play in the modern-day clinical trial and are you future-ready?
The impact of evolving team roles
Over recent years we have also witnessed the evolution of traditional functional roles, the creation of new roles and the blending of others. It is now widely recognised that everyone needs access to the same data, albeit from varying lenses including Medical, Clinical Operations (SM/Monitoring), Data Management and Biostats.
There has also been a shift from a siloed functional approach to a more integrated study team approach. Additionally, to take advantage of advances in technology, the skill sets and capabilities for all team members needs to evolve.
The importance of effective oversight
Whilst this is a challenge, there’s a huge opportunity, and a potential competitive advantage, for companies who are able to leverage, analyse and visualise the emerging data. Whether the trial is being run in a traditional bricks and mortar style, or in a hybrid setting, having a single, standardised, integrated and accurate source of information at your fingertips is crucial.
Creating near real-time actionable insights for the whole study team, irrespective of function, requires a holistic approach to collecting and leveraging both internal and external data.
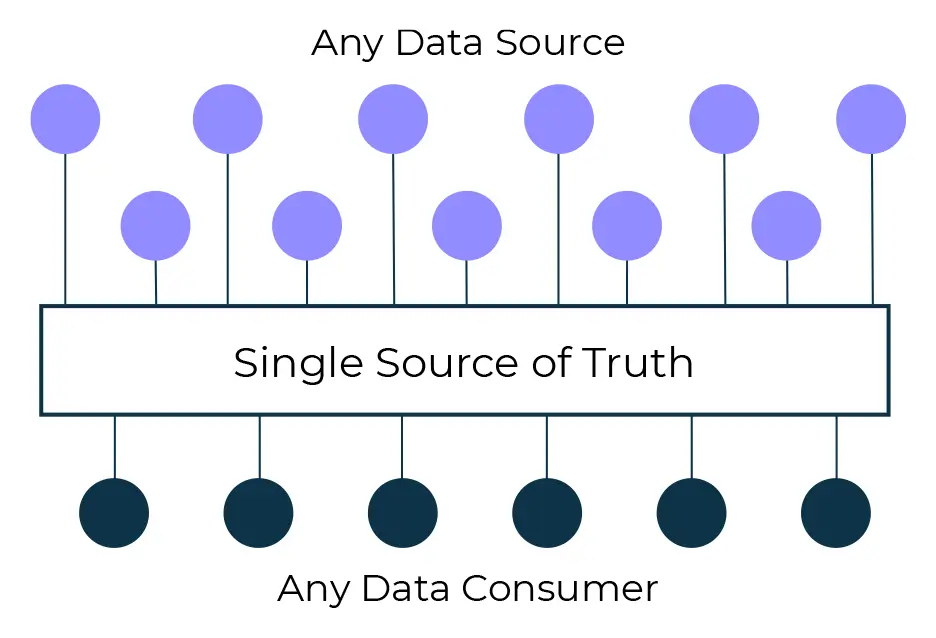
Near real-time Actionable Insights
The value of creating actionable insights
Generating actionable insights from a single source of truth when designing, planning, executing and reporting clinical trials, is becoming a real game changer.
For example, when the pandemic started we were in a very dynamic and uncertain environment. Clinical and Operational teams needed to proactively manage their ongoing and planned studies, based on differing Covid-19 prevalence rates, within and across countries. They needed to access their internal clinical trial data platforms AND the emerging external prevalence data. A good illustration was when sponsors were able to combine data and act on the insights revealed e.g.
- Externally: e.g. from Johns Hopkins prevalence/infection rates, it was possible for biostatistics and clinical operations to partner and develop models to understand and predict the impact on trial delivery.
- Internally: e.g. dynamic COVID-19 oversight/planning & reporting dashboard, site and country level reporting e.g. patient safety, IP, monitoring/SDV (remote), digital solutions were needed.
Using powerful, and agile, clinical operations data platforms which generate advanced analytics has proven essential in ensuring trial continuity, reducing recruitment delays AND to ensure that digital engagement solutions are having the desired impact.
Another illustration of the clinical and operational analytics and insights is in protocol design and delivery. It is now possible to proactively leverage data informatics and patient insights to drive more data derived discussions when developing trials (e.g. using competitive landscaping, disease prevalence, patient burden, historical performance, referrals, claims data, patient registries, biomarkers etc.). Using such analytics can evaluate the study viability, highlight opportunities for simplification, and explore where decentralized solutions can be used to decrease patient burden etc. Here the ROI can be significant by avoiding costly amendments. Actively managing the End-2-End workflow is another ripe area for leveraging technology.
From a medical perspective, monitoring patient safety is of paramount importance. Using centralized monitoring and having the ability to visualize multiple data points can help identify unexpected safety signals. Overlaying this with AI and ML will change the way we monitor patient safety, which is the ultimate ROI.
Key take away messages
Ensuring appropriate oversight of clinical trial data, risk-based strategies and reliable operational and clinical analytics, is becoming more critical than ever for trial success. You must be able to trust the data you are using to make crucial decisions.
Agile protocols require agile clinical data platforms to ensure robust oversight and manage risk appropriately.
Ensure your Clinical Data Platform is fit for purpose and has the flexibility to take advantage of future advances in AI, robotic process automation and ML.
Read more in a new white paper on ‘Moving Beyond Traditional CTMS to Unlock Value in an Outsource Model’
Access the White Paper
By submitting, you agree to the processing of your personal data by eClinical Solutions as described in our Privacy Policy.
Author

Jason Gubb has more than 25 years of experience in global clinical operations strategy and leadership. He has an applied knowledge of leveraging internal and external data, analytics, digital technology and collaborative partnerships to create actionable insights for study teams. Mr. Gubb works with startups, pharma, vendors and contract research organizations (CROs) to develop innovative approaches to optimize protocol designs, modernize clinical trial conduct and accelerate trial delivery.







