
The Future State & Evolution Needed to Accelerate Digital Transformation
Key Takeaways from eClinical Solution’s Society for Clinical Data Management (SCDM) Global Conference Sessions
During the SCDM global virtual conference this year, experts from eClinical Solutions joined industry leaders in conversations about the current and future state of the evolving life sciences industry. From accelerating decentralized clinical trials adoption to the evolution from clinical data manager (CDM) to clinical data scientist (CDS), each eClinical Solutions session strived to inform and inspire attendees with ways to drive change and harness the advancements of digital technologies.
Advancing Decentralized Clinical Trials Adoption
A pressing issue among many life sciences professionals adjusting to a post-pandemic reality is how to accelerate decentralized clinical trials (DCT) adoption effectively and securely. While identifying and filling the gaps in technology that are stalling DCT adoption is a critical step, eClinical’s Chief Marketing Officer, Sheila Rocchio sees the approach to widespread decentralized trials as two-fold.
During a rapid-fire discussion on the topic with leaders from Boehringer Ingelheim, Veeva, Medidata, and Castor, Sheila explained, “Sponsors will be the most influential in making DCT adoption a reality, if we as an industry and the sponsors are writing protocols with the site ecosystem and participants in mind. The reason it’s taken so long to get here is not only a technology problem, but also an industry challenge to create a different research model.”
While the path towards establishing greater DCT adoption may be long, modern clinical data platforms are rising to the challenge. The use and adoption of patient-centric decentralized platforms continues to increase in clinical research. As more companies incorporate decentralized trial models and technology, clinical data platforms like elluminate® can help accelerate DCT strategy and rollout.
eClinical’s Dawn Kaminski, Senior Director Data Strategies and co-chair of the SCDM conference, demonstrated alongside Sales Engineer Achilleas Zaras how one centralized view of data for review, analytics and oversight is critical for advancing decentralized trials. Dawn elaborated on the benefits of a clinical data platform that is system agnostic during the Product Showcase Q&A, stating that “elluminate can ingest data from pretty much any system, including major EDC systems, central labs, IRT, and wearable systems, as well as financial and clinical trial tracking data. Data review can be done whenever you need to, because data is constantly coming into the system and being refreshed.”
The ability to assign data listings to different review roles, either within a single study or across studies, and then also restrict or allow certain organizational roles to add queries or issues during data review is another way platforms like elluminate can help alleviate the data review burden of decentralized trials. As the industry moves towards a goal of wholly decentralized clinical trials, it’s clear that a comprehensive & flexible clinical data platform is the necessary foundation for sponsors to build upon.
Fostering Digital Transformation in Clinical Data Services
The life sciences industry is currently at a pivotal point where new methods, technologies, and ideas from other industries are increasingly contributing to a revolutionary age in biotech. Ideas such as omics data, wearables, streaming digital solutions, enhanced Imaging, metadata-centric tooling, Machine learning (ML), Artificial intelligence (AI), Internet of Things (IOT), and Robotic Process Automation (RPA) combined with a vendor industry developing technology solutions at an unprecedented rate makes for an exciting but confusing mix of opportunities.
During the session, “Fostering Digital Transformation in Clinical Data Services – Can it spur and support change?”, eClinical CEO Raj Indupuri discussed with experts from AstraZeneca, Veeva, and Novartis how reimagining current data infrastructures will lead to a faster modernization for clinical trials with digital transformation taking place at the enterprise and operational level. Raj explains, “To truly achieve clinical trials modernization, the current data infrastructure in siloed systems will not work. From my perspective, modernization requires building a strong technology foundation, and a focus on automating and modernizing the different components of a clinical trial and the clinical trial data life cycle.”
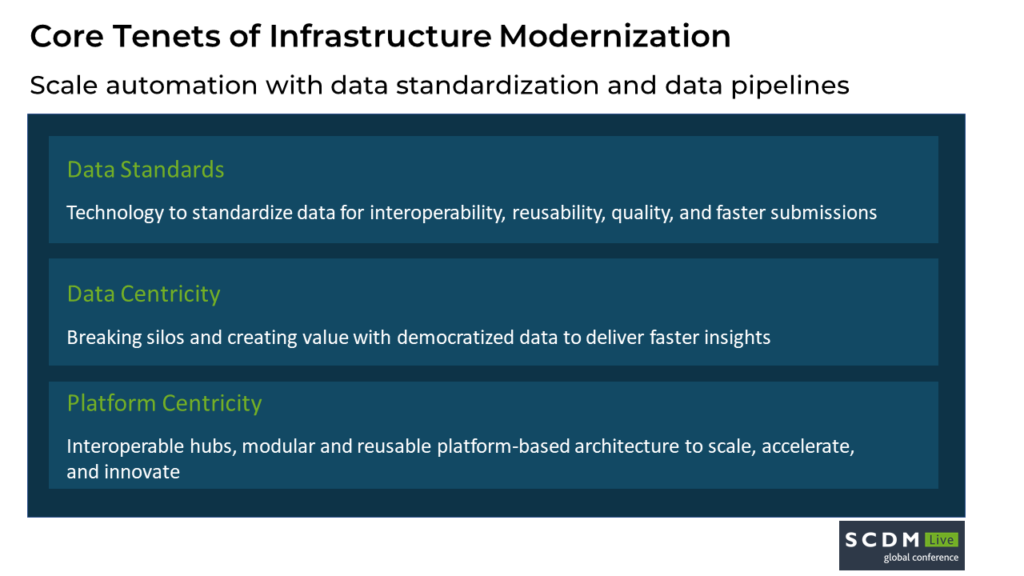
Raj further elaborates on how breaking the siloed data infrastructure is critical to ensure all data is delivered to the right people at the right time. A platform-centric approach is key when examining digital transformation capabilities, with an interoperable hub enabling greater oversight and control over clinical data as it moves from different business units.
The Evolution from Clinical Data Manager (CDM) to Clinical Data Scientist (CDS)
Technology advancements in clinical research are impacting life sciences organizations at a role-based level, specifically as a catalyst for transforming the role of the Clinical Data Manager to Clinical Data Scientist.
In this presentation, eClinical’s Dawn Kaminski and Erin Broderick, Data Cleaning Lead at GlaxoSmithKline, explored how far data review strategy has come in recent years and what improvements data managers and scientists can look forward to in the future.
As Dawn took us in her virtual time machine back to the year 1999, she showed how the prior methods for data review were extremely labor intensive. She explains how “many hours were required by data managers facilitating review, programming and manually creating the listings that were reviewed, and quality checking to ensure the programs that were specified were really hitting on the data points that data management wanted to focus on.” Reconciliation across various data sources was also challenging, because the process was completely siloed.
Dawn continues to examine the gradual shift in data review in recent years, having seen an increase in the focus on artificial intelligence and machine learning to better assist in trend and issue identification. These advancements allow data managers to hone in on specific conditions and find “the needle in the haystack” much more quickly than in previous years. Utilizing machine learning in clinical trials can help identify data trends and predict them in certain outcomes more efficiently than with traditional methods.
The result of this shift is more streamlined and targeted review that allows data managers to spend less time on manual work, and more time forming actionable data insights rather than handholding every step of oversight and facilitation.
Cultivating a Personal Brand for the Clinical Data Manager
As every life science organization undergoes digital transformation initiatives and process modernization, they are looking for seamless flows of data, advanced analytics, and management that adds value while delivering high-quality data streams for new insights and therapies. This combined with the move to decentralized trial models that rely on more data sources results in increased pressure on data management to do less with increased quality expectations and cycle times.
Katrina Rice, eClinical’s Chief Delivery Officer of Data Services, notes how the Clinical Data Manager role must evolve to accommodate this wave of advancement in the panel discussion, “Keeping It Real: Personal Brand Success in the Age of COVID-19 is about Self-definition, Transparency, and Authenticity”.
Katrina emphasizes that a well-developed personal brand can make the advice as a Clinical Data Manager more authoritative and help establish a position as a thought leader with unique and valuable insights as organizations continue to grow. She views the CDM role as needing to evolve into a Strategist that can truly own the data that’s rapidly coming in from these various sources, better understand the details of the clinical data flow, and establish themselves as strategic change agents who can look at existing processes and help employ methods to improve them.
She explains, “If data is the currency of life sciences, then isn’t it more important than ever for data managers to claim their seat at the table as not only managers of the data, but true data strategists?” She recommends that CDMs take the initiative to participate in and develop teams, look into process improvements, fully seek out leadership opportunities, and be flexible in moving in different directions as companies deploy digital transformation initiatives in order find their place at the table.
Looking Toward a Digitally Transformed Future
Technology’s role in the life sciences industry only continues to expand, and the SCDM global virtual conference captured the critical developments needed to aid in this digital evolution. The future of clinical research is dependent on role-based process improvements as well as enhanced data strategy through the use of modern, flexible platforms like elluminate that can provide visibility, traceability, transformation and analysis as the industry moves forward.
Author

By submitting, you agree to the processing of your personal data by eClinical Solutions as described in our Privacy Policy.






