
3 Ways to Enable Better Collaboration Between Your Clinical Trial Teams
Simply put, clinical trials are complicated. This comes as a surprise to no one, but research indicates that there is no end in sight for trial complexity in the coming years. Tasked with handling a greater influx of data while innovating new ways to support patient registration and participation has left some clinical trial teams scrambling to implement effective solutions.
Counteracting trial complexity, both today and down the road, will require clinical study teams to work together in order to make the right decisions based on their own individual circumstances. But in light of the pandemic, in addition to the rising number of clinical operations systems being utilized, clinical trial teams are increasingly working in silos. Naturally, a disparate team all working on their own initiatives within black boxes makes it hard to know who’s responsible for what, and how trial issues are being addressed.
Let’s take a closer look at how clinical operations teams can foster better collaboration between their clinical trial teams.
Establish a Game Plan for Your Operating Model
Your preferred operating model is a critical component of your overall development strategy and while it may change as you grow it’s also a core of your company culture. Do you prefer one large CRO that can handle all your needs or enjoy working with focused providers that are experts in certain aspects of clinical development. There is also a hybrid approach to build internal teams that develop process and oversight early on where some companies wait until a critical mass of trials to handle work internally. Operating model philosophy usually stem from previous industry experience with all of the various models along with your specific indication and patient population. The most common operating models selected:
- One full service CRO – The proverbial – one company, one contact and one escalation path if things go awry is what this model delivers, as well as a team that all works within one organization. Benefits include simplicity and scale and at least seemingly less work for the sponsor who does not have to handle numerous providers. Cons include service levels and attention, constant turnover and the potential of your trial getting less attention than the “big guys” that may be competing for top resources.
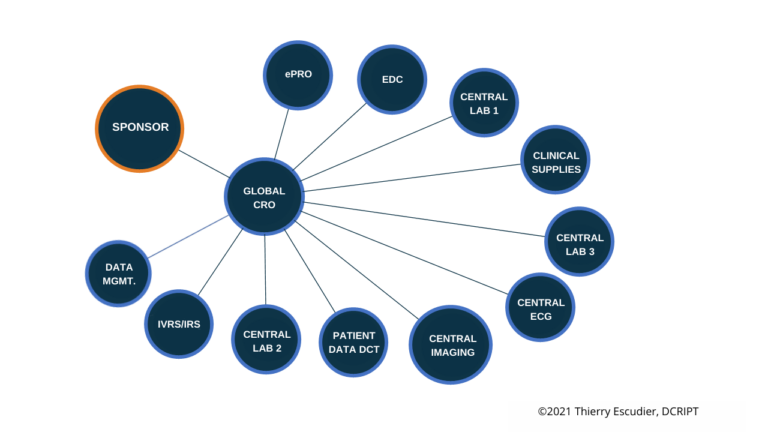
- Best of Breed Specialty – Working with smaller providers that have a focus in certain areas such as selecting clinical providers separately from data services is another approach that typically leverages smaller providers with more depth knowledge in their area of expertise. The pros include better attention to quality and service levels overall, along with specialized knowledge of your team and trial and the cons include more work on the sponsor to manage multiple trials and being the hub of communications.
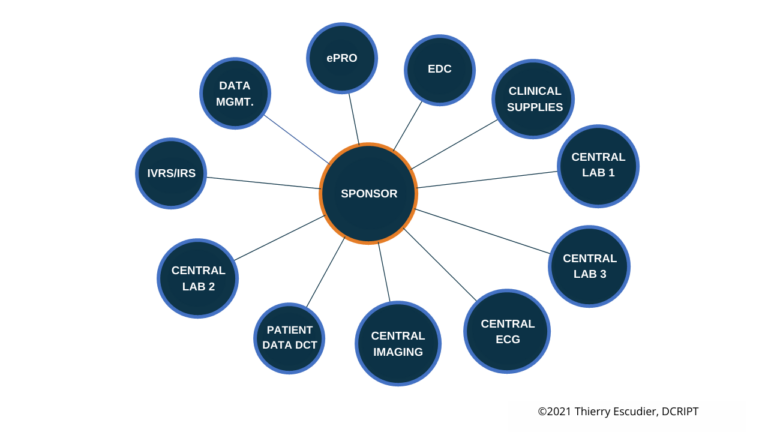
- Hybrid – There is a hybrid model which utilizes a distributed operating model that has certain responsibilities owned by your internal teams and other responsibilities outsourced to a CRO. But even opting for the best-of-both-worlds approach requires everyone within your organization to be aligned on who will be responsible for what.
Ultimately, determining which operating model will work best for your particular trials and teams will take time and patience. There’s not one option that’s better than the rest, so your decision will need to take into account the best interests and preferences of all those involved. Considering the ramifications and benefits of prospective models, as well as the ownership elements of each model, will help you make the right decision and get your clinical trials started off on the right foot.
Work from a Centralized Source of Truth
Working from the most current, accurate data is a must for your clinical trials. Executive leadership will often ask your teams about patient enrollment, data access, and trial insights, and finding the answers to those questions quickly isn’t always a straightforward process.
Pressure from above can put stress on the system, and while no one wants to pass along the wrong information and lose credibility, being confident that you’re sharing the right information requires everyone to be working from a centralized source of truth. Having a shared platform that is consistently being vetted, updated, and organized can provide much-needed peace of mind to clinical trial teams that need to agree on a single source of truth.
Seek out clinical and operations software that consolidates disparate data sources and trial information so that team members don’t have to spend hours tracking down a particular version they need from internal or external teams. Datasets are becoming increasingly complex and numerous in today’s world, which makes it all the more important to ensure accurate, credible data can be accessed, analyzed, cleaned, and reported on in a timely and efficient manner.
A centralized source of truth works to break down siloed processes and foster better collaboration between teams that need to ensure quality and validity throughout every step of a clinical trial.
Take a Risk-Based Approach to Problem-Solving
A centralized platform not only works to streamline clinical trial processes and ensure the reports you submit can be backed up, but it also helps mitigate risks associated with global regulations compliance.
Achieving the outcomes your clinical trial teams are interested in requires you to analyze existing clinical operations to identify issues that could cause problems. Adhering to a risk-based approach to problem-solving and identifying upfront what constitutes risk will make it easier to avoid them down the road.
Within a cross-functional team, understanding who is working on any given problem or issue within the clinical trial ensures efforts aren’t being duplicated. Centralizing information around troubleshooting issues and risk mitigation gives much-needed visibility and traceability into the work being done, the actions being taken, and the status of outstanding problems.
Running effective clinical research starts with identifying early on where problems may arise and proactively acting to prevent them. With a risk-based approach to problem-solving that’s supported by a centralized source of trial information, your teams can operate confidently and efficiently knowing the accurate status of ongoing work.
Enable Better Collaboration with the Right Technology
Better collaboration on your trials starts with understanding what systems and operating models are going to best support the work you’re doing. Regardless of the structure, a centralized clinical and operational data platform will provide your teams with peace of mind knowing all data assets are consolidated, traceable, accurate, and accessible. The value provided by having a single source of truth to work from cannot be overstated, both from a data quality standpoint as well for assurance of regulatory compliance and oversight.
For clinical trials looking to streamline and simplify their clinical operations processes, the elluminate® Clinical Data Cloud can help. We exist to make clinical research data acquisition and analysis easy and intelligent in order to help your teams bring new treatments to patients faster. Together, we can work to enable the efficient collection, standardization, reporting, and role-based utilization of clinical research data within your organization.

The Clinical Development Digitization Guide, Strategies to Drive Success
Take a deeper look into the key components of preparing for the digitization of today’s clinical trials in this new white paper. You will learn the necessary steps that organizations must take to embrace the transformation of processes that will help usher in a new way forward for the industry.
If you want to see the elluminate Clinical Data Cloud in action, contact us below or request a demo today.
By submitting, you agree to the processing of your personal data by eClinical Solutions as described in our Privacy Policy.







