By submitting, you agree to the processing of your personal data by eClinical Solutions as described in our Privacy Policy.

30-Minute Academy Webinar
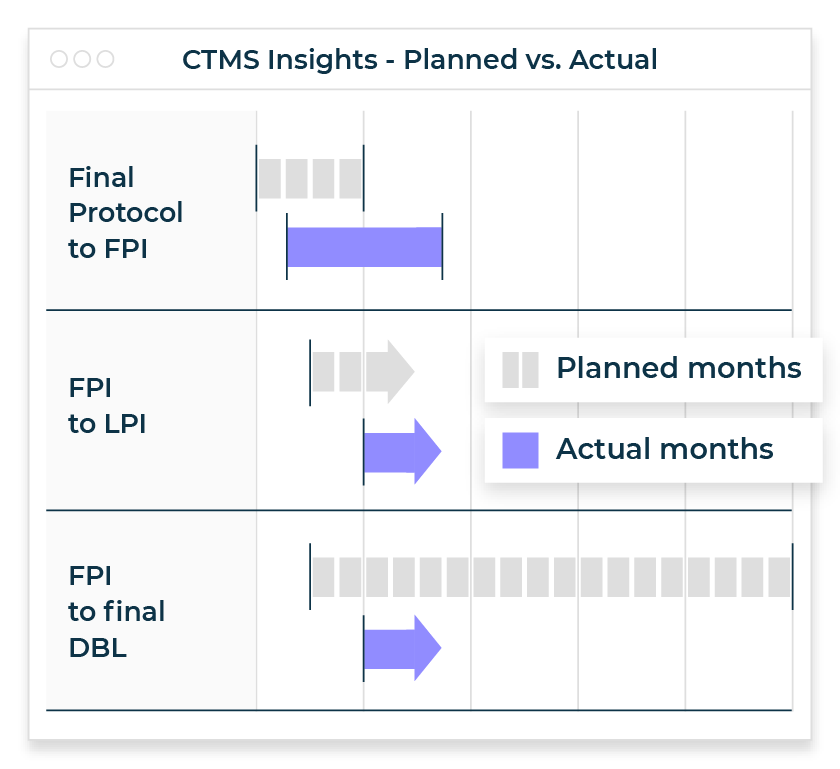
Comprehensive operational data is essential for any life sciences company, but with the increase in outsourced providers and partners along with the jump in decentralized trials, getting access to this information can be a challenge. This critical data is owned by clinical research organization (CRO) partners and organizations have had limited options for gaining these insights — until now.
elluminate® CTMS Insights provides ONE centralized place for all your operational data. It is designed for organizations looking to own and mine their operations data for performance metrics without relying on downloads and spreadsheets.
If you are looking to scale through data-driven operational excellence, join us for this 30-minute webinar to learn how elluminate CTMS Insights:
- Provides on-demand operational insights, analytics, and visualizations for proactive trial oversight and management
- Leverages the elluminate Clinical Data Cloud to automate ingestion from a wide variety of sources including CRO’s CTMS systems for companies working in outsourced models
- Provides portfolio metrics and dashboards without relying on manual updates from your CTMs for decision-making and planning
Who Should Attend
- Life sciences companies of all sizes working in outsourced models
- Clinical operations leadership
- Clinical trial managers
- Development leaders
- Clinical Informatics leaders
- Research and development professionals
- Research and development analytics teams
Presenter

As a Solution Consultant, Jason works with Sponsor organizations to understand their challenges and come up with creative solutions leveraging the elluminate platform. Jason has lead design & development for several products ranging from a Data Management Workbench, Data Integrations, Clinical Operations Platform, Clinical Analytics, and Risk Based Quality Management. Jason has been in the clinical trials industry for over 15 years and his prior experience includes, global central laboratory, eCOA design, process improvement, and building an automated workforce.