Download Your Copy
By submitting, you agree to the processing of your personal data by eClinical Solutions as described in our Privacy Policy.


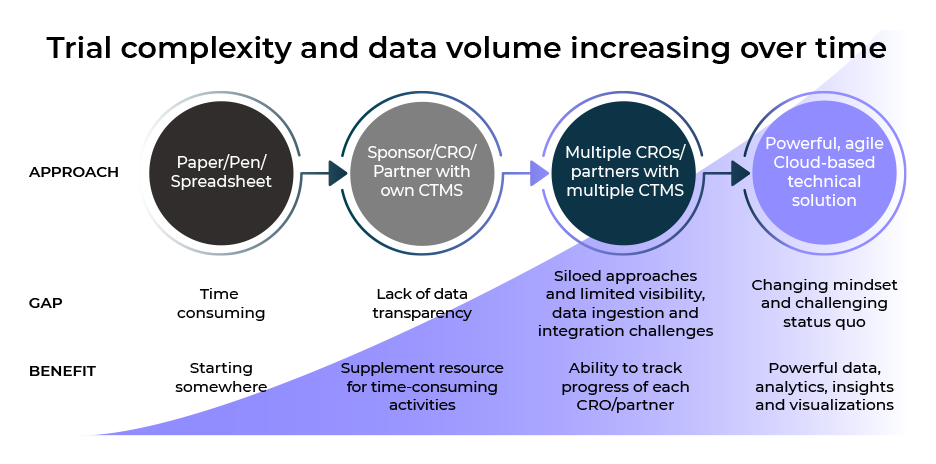
The traditional Clinical Trial Management System (CTMS) was designed to view and keep data within the walls of either the sponsor, the Clinical Research Organization (CRO) or data vendors. Historically a CTMS was able to sufficiently handle clinical trial data as there were fewer data sources. However, the proliferation of data sources and the rise of outsourced trials changed how CTMS is defined and called into question whether they are fit for purpose in today’s world.
This new white paper unpacks the evolution of CTMS and its role in modern-day clinical trials. It highlights how to chart the best path forward in the growing complexity of the outsourced model and how to ensure appropriate oversight of clinical trial data and risk-based strategies so you can ultimately trust the data to make crucial decisions.
To download the complete white paper and learn more, please Fill out and submit the form on this page.